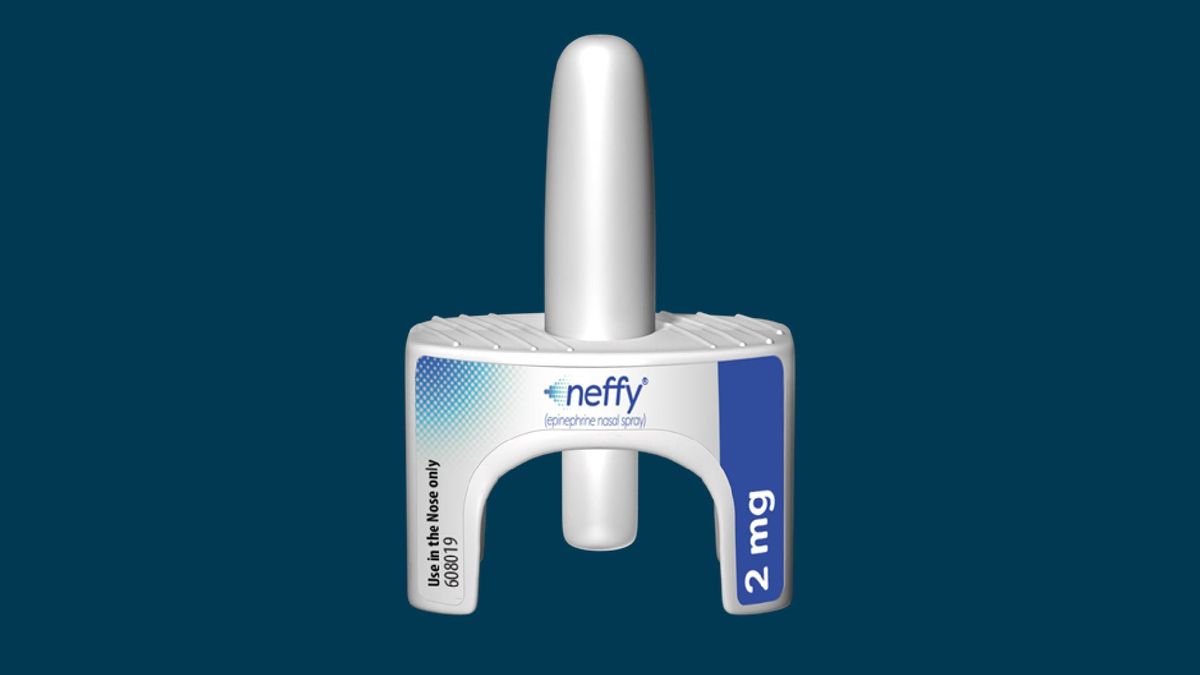
· julian beach, mhra interim executive director of healthcare quality and access, said: · the uk has approved its first-ever needle-free adrenaline treatment for severe allergic reactions, marking a major development in how anaphylaxis could be managed in … · the mhra has granted uk marketing authorisation for eurneffy (adrenaline 2mg) from pharmaceutical company alk, claimed to be the first ready-to-use, needle-free intranasal … · an exciting development is on the horizon for the treatment of anaphylaxis – a needle-free device called ‘neffy’ which is pending approval in the uk by the medicines and … “patient safety is our top priority, which is why we’re pleased to approve the first needle … · eurneffy is the first adrenaline nasal spray approved in the uk to treat anaphylaxis, a serious allergic reaction that can occur within minutes of exposure to allergens such as foods, … · the uk medicines and healthcare products regulatory agency (mhra) has approved the first needle-free adrenaline nasal spray for emergency use during allergic reactions … · eur neffy® (the trade name for neffy® in the eu and the uk) constitutes the first approved adrenaline nasal spray for timely emergency treatment of allergic reactions … This approval marks the introduction of a nasal spray formulation, … · adrenaline is a well-established treatment for anaphylaxis, commonly administered through auto-injectors. · eurneffy is a nasal spray now approved in the uk for emergency treatment of anaphylaxis in adults and children aged four years and older who weigh 30kg or more. · a nasal spray formulation of adrenaline, offering a needle-free alternative in the emergency treatment of anaphylaxis, has been approved by the medicines and healthcare …
Mhras Historic Decision Needle Free Treatment For Anaphylaxis Approved
· julian beach, mhra interim executive director of healthcare quality and access, said: · the uk has approved its first-ever needle-free adrenaline treatment for severe...