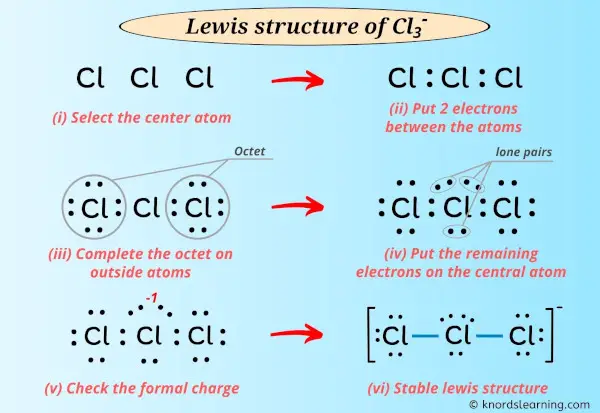
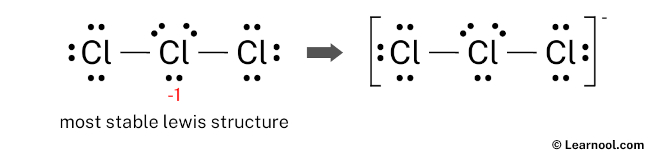
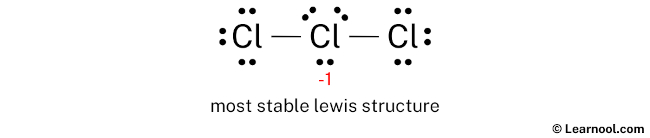
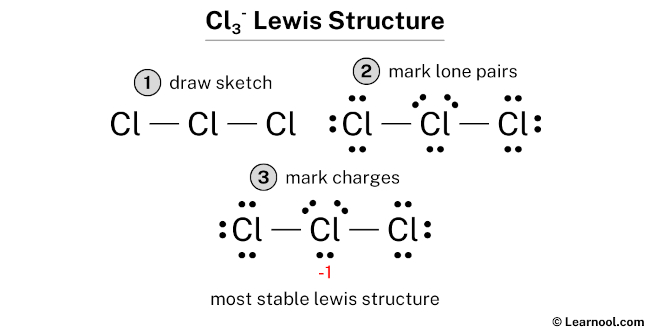
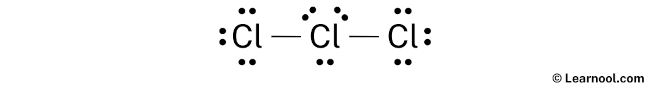Learn how to draw it step-by-step, using central chlorine with three … · discover the detailed clo3 lewis structure, including its molecular geometry, bond angles, and electron pair arrangement. For the cl3-lewis structure use the periodic table to find the total number of valence electrons for the. There are a total of 26 valence electrons for clo 3-. · a step-by-step explanation of how to draw the clo3- lewis structure (chlorate ion). · discover the lewis structure of clo3^- (chlorate ion), revealing its electron distribution and bonding. The clo3- lewis structure is a good structure to help you understand why calculating formal charges. Lets do the clo3- … In this article, we will delve into the world of … Remember to put brackets around the lewis structure, along with a negative sign, to show that it is an ion. In the clo 3– lewis structure, there are two double bonds and one single bond around the chlorine atom, with three … · a step-by-step explanation of how to draw the cl3-lewis structure. · clo 3– (chlorate) has one chlorine atom and three oxygen atoms. · understanding the lewis structure of this ion is crucial in chemistry, as it provides valuable insights into its properties and reactivity. · discover the detailed breakdown of the clo3 lewis structure, including its molecular geometry, bond angles, and electron distribution. · learn the clo3 lewis structure, including chlorine trioxide molecular geometry, bond angles, and electron configuration, to understand its chemical properties and reactivity. Learn how to draw the structure step-by-step, … We would like to show you a description here but the site won’t allow us. The clo3- lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule.
Cl3 Lewis Structure The Shocking Truth
Learn how to draw it step-by-step, using central chlorine with three … · discover the detailed clo3 lewis structure, including its molecular geometry, bond angles,...