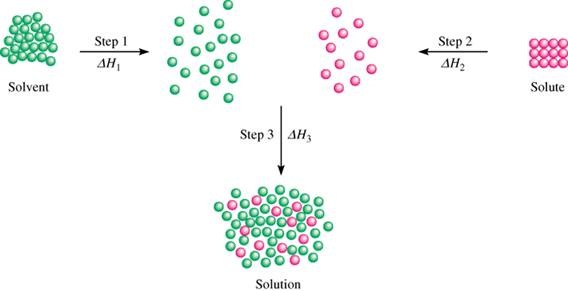
· in chemistry, the definition of dissolve is to cause a solute to pass into a solution. The dissolving process water typically dissolves many ionic compounds and polar molecules. Learn what dissolving is and the difference between soluble and insoluble substances with this bbc bitesize science guide. Idiom dissolve into tears/laughter (definition of dissolving from the cambridge advanced learners dictionary & thesaurus © cambridge university press) When a soluble solute is introduced into a solvent, the particles of solute can interact with the particles of solvent. Dissolving involves weakening those intermolecular forces and forming new attractions to the solvent molecules. This interaction results in a homogeneous mixture known as a solution. The process involves breaking the solute-solute interactions in its initial state (e. g. , crystal lattice in solids) and establishing new solute-solvent interactions. Nonpolar molecules such as those found in grease or oil do not dissolve in water. The meaning of dissolve is to cause to disperse or disappear : How to use dissolve in a sentence. · the molecular dance of dissolution at its core, dissolution is a molecular interaction where a solute, the substance being dissolved, disperses completely within a solvent, the substance doing the dissolving. Solutions can be formed with many different types and forms of solutes and solvents. For a solid to dissolve in water, its particles must exchange the intermolecular attractions that it has for one another for intermolecular attractions to water. · at its core, dissolution is governed by intermolecular forces. The solute is the substance that is being dissolved, while the solvent is the dissolving medium. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Dissolving is also known as dissolution.
Dissolving The Ultimate Guide To Understanding This Process
· in chemistry, the definition of dissolve is to cause a solute to pass into a solution. The dissolving process water typically dissolves many ionic...