Since inspections of manufacturers of active substances are based on risk,. Data demonstrated rapid symptom relief and attack resolution regardless of attack. You can look for any word, phrase or product licence number (pl) using the search tool. Under a joint information sharing agreement, pharmaceutical companies will be invited to register early with the mhra and nice to allow parallel decision making over licencing and value. & salisbury, england– (business wire)– kalvista pharmaceuticals, inc. · come and join the next mhra symposia in february 2025 and hear the latest on good clinical practice (gcp) and good laboratory practice (glp) in the uk from mhra inspectors and regulators. Kalv) today announced that the medicines and healthcare products regulatory agency (mhra) of the united kingdom (uk) has granted marketing authorization for ekterly ® (sebetralstat), a novel plasma kallikrein inhibitor, for the … Recognized globally as an authority in its field, the agency plays a leading role in protecting and improving public health and supports innovation through scientific research and development. · cambridge, mass. · first new on-demand hae treatment in over a decade, with potential to transform management of the disease. Manufacturers, importers and distributors of active substances are required to register their activities with the mhra. You can also use the a-z list to find the active substance. · the medicines and healthcare products regulatory agency regulates medicines, medical devices and blood components for transfusion in the uk. · the medicines and healthcare products regulatory agency (mhra) website is now on gov. uk. As part of the move to gov. uk … The medicines and healthcare products regulatory agency (mhra) is an executive agency of the department of health and social care in the united kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe. The medicines and healthcare products regulatory agency (mhra) regulates medicines, medical devices and blood components for transfusion in the uk. The mhra products website allows you to find:
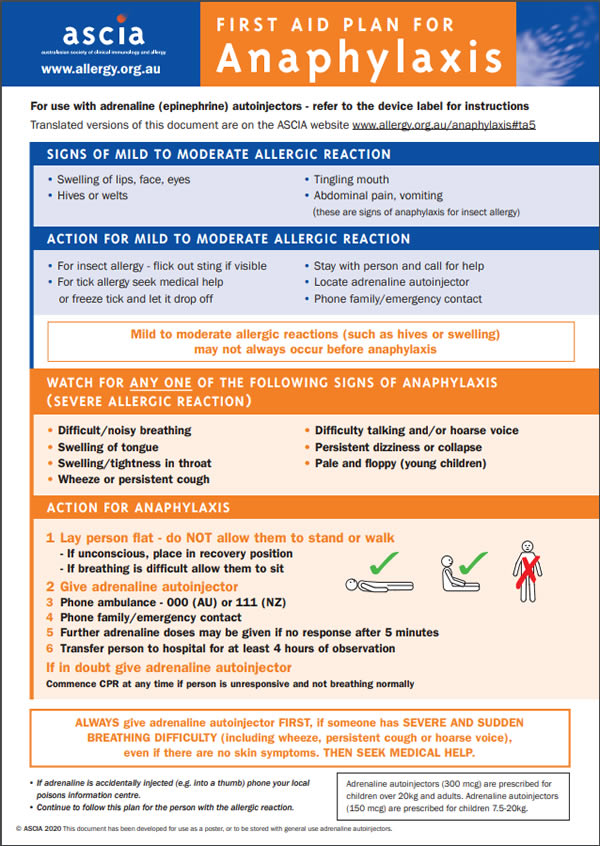
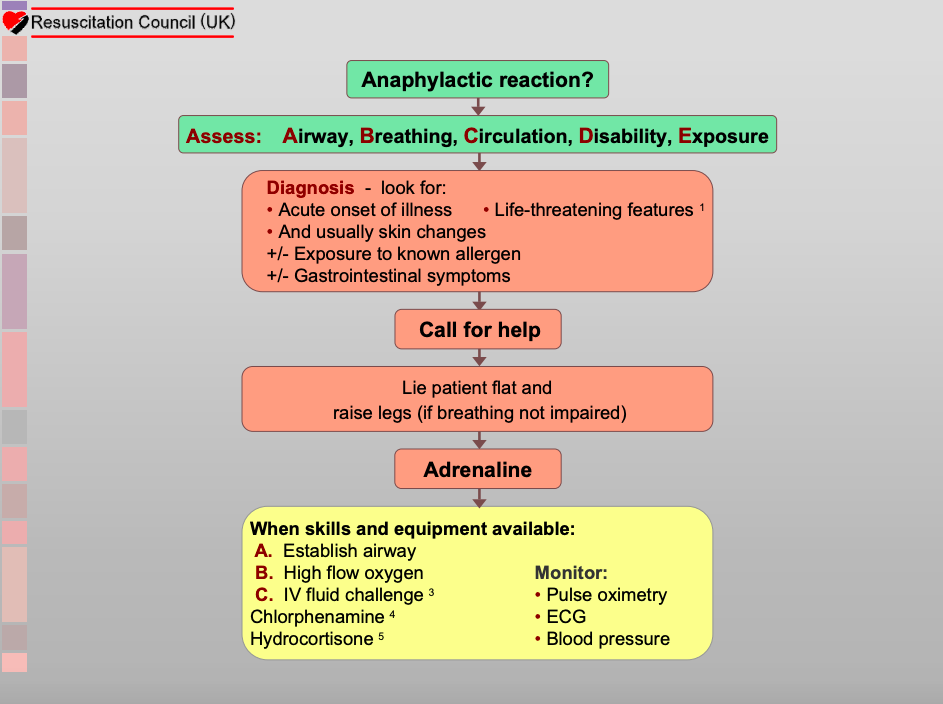
Mhra Approves First Needle Free Emergency Anaphylaxis Treatment What You Need To Know
Since inspections of manufacturers of active substances are based on risk,. Data demonstrated rapid symptom relief and attack resolution regardless of attack. You can look...