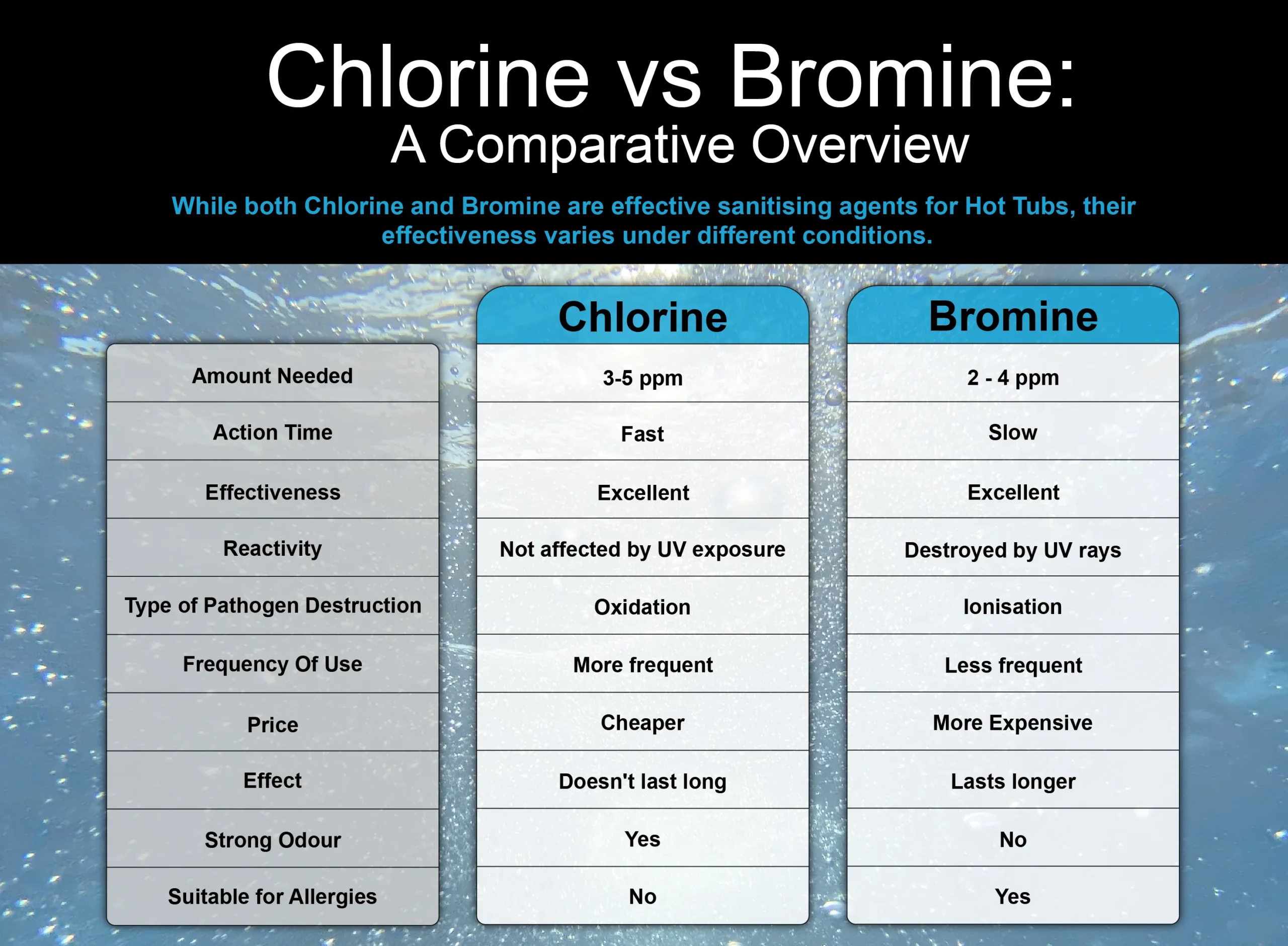
· chlorine chemistry helps keep drinking water and swimming pools safe, and is used to manufacture household chlorine bleach, which can whiten and disinfect clothes and … At room temperature, chlorine is a yellow-green gas that is heavier than air and has a strong irritating odor. It is also the second most abundant element in the human body after oxygen. · chlorine is a highly poisonous, greenish yellow gas, about two and a half times as dense as air, with a strong, sharp, choking odor. · the element chlorine is the halogen located in group 17 in the periodic table. Denoted by the chemical symbol cl, it categorized as a nonmetal [1, 2]. Chlorine is the chemical element that constitutes about 0. 017 percent of the earth’s crust. It was, in fact, one of the first poisonous gases used in warfare—in 1915 during world war i. Element chlorine (cl), group 17, atomic number 17, p-block, mass 35. 45. It can be converted to a liquid under pressure or cold temperatures. Chlorine is a greenish - yellow , diatomic , dense gas with a sharp smell (the smell of bleach). Scheele knew that chlorine was a new element, but thought it contained oxygen as well. Chlorine is a greenish - yellow poisonous gas. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Much chlorine is used to sterilize … · chlorine, chemical element of the halogen group that is a toxic, corrosive, greenish yellow gas, irritating to the eyes and respiratory system. This ai-generated answer is powered by openai. It was discovered in 1774 by swedish chemist carl wilhelm scheele (1742-86). · chlorine , chemical element of the halogen group that is a toxic, corrosive, greenish yellow gas, irritating to the eyes and respiratory system. Creating an answer for you using ai. Chlorine is a chemical element; As a common disinfectant, elemental chlorine and chlorine-generating compounds are used more directly in swimming pools to keep them sanitary. It has symbol cl and atomic number 17. Chlorine is mainly used as bleach in the manufacture of paper and cloth and to make a wide variety of products. · chlorine is an element in the halogen group that is a pale yellow gas at room temperature and pressure. Chlorine is a greenish-yellow gas at room temperature, known for its powerful disinfectant properties and pungent odor. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. It is the third most abundant element in the earth’s crust and is the most abundant halogen. Chlorine appears as a greenish yellow gas with a pungent suffocating odor. Readily liquefied by pressure … · chlorine is a reactive gas that is approximately three times as heavy as air and has a characteristic odour similar to bleach. Chlorine is a greenish - yellow gas at room temperature, known for its powerful disinfectant properties and pungent odor. It is not found free in nature as it combines readily with nearly all other elements. Ai-generated content may sometimes contain inaccurate, incomplete, or biased information, so make sure you do additional research. Slightly soluble in water. As a member of the halogen group, it is highly reactive, especially with hydrogen, forming hydrochloric acid, a substance fundamental to various industries. Chlorine is a member of the halogen family. Elemental chlorine at high concentration is … It was, in fact, one of the first poisonous gases used in warfare — in 1915 during world war i (1914 – 1918). · what is chlorine? Toxic by inhalation. As a member of the halogen group, it … Chlorine is a yellow-green gas at room temperature. Its chemical symbol is cl, and it has an atomic mass of 35. 453. It is part of the group of chemicals called ‘halogens’ … The different chemicals may react and release chlorine gas into the air of … You should not rely on this feature for medical, financial, or legal advice. Much chlorine is used to sterilize water and wastes, and the substance is employed either directly or indirectly as a bleaching agent for paper. · chlorine poses a danger in your home when you mix a chlorine-based cleaner with another cleaner. Chlorine (pronounced as klohr-een) is a highly reactive diatomic gas belonging to the family of halogens. Liquefies at -35 °c and room pressure. Chlorine is a highly poisonous, greenish yellow gas , about two and a half times as dense as air, and with a strong, sharp, choking odor.
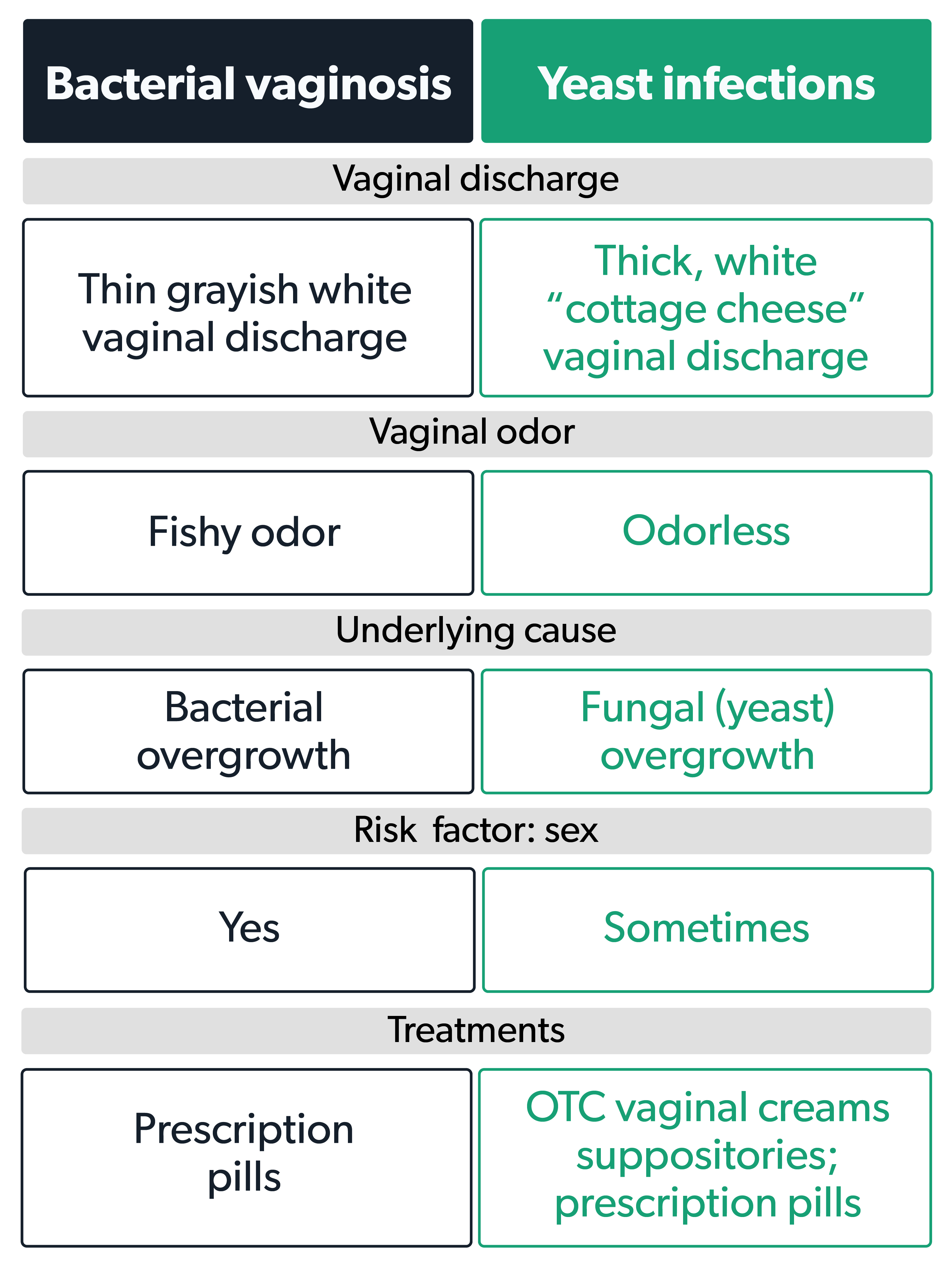
Chlorine Vs. Yeast Infections: What You Need To Know
· chlorine chemistry helps keep drinking water and swimming pools safe, and is used to manufacture household chlorine bleach, which can whiten and disinfect clothes...