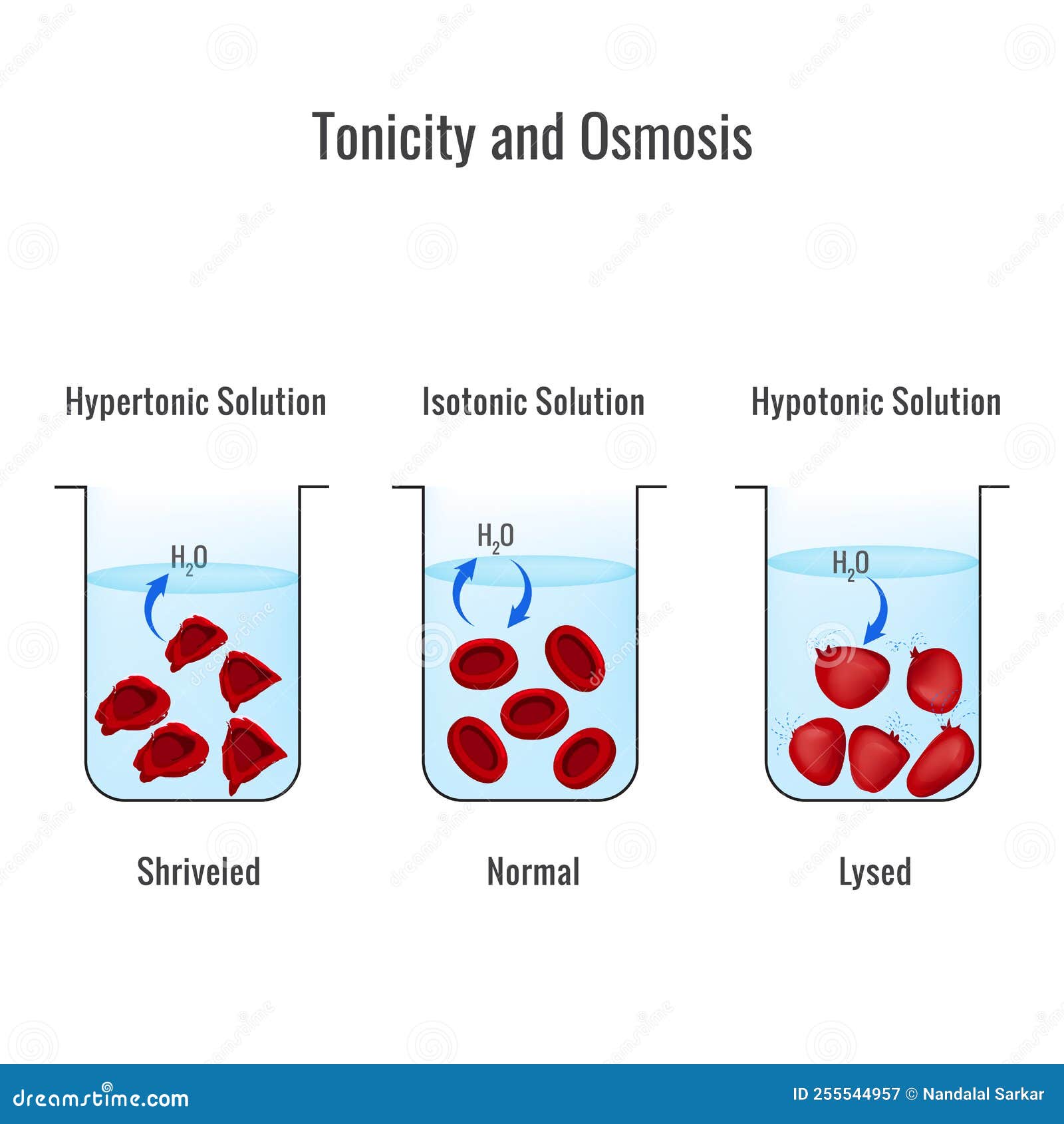
Osmosis is a passive process and happens without any expenditure of energy. It involves the movement of molecules from a region of higher concentration to lower concentration until the … Osmosis is the phenomena in which solvent molecules pass through a semi … Theory what is osmosis? Osmolarity is the expression of osmolar concentration. The passage of water across a selectively permeable membrane is known as (a) osmosis (b) diffusion (c) facilitated diffusion … Osmolality and osmolarity are expressions of concentration reflecting the osmoticity of solutions. The number of moles of solute per unit volume of solution is measured by molarity, while the number of osmoles of solute particles per unit volume of solution is measured by osmolarity. Diffusion and osmosis chemistry questions with solutions q1. Aim to study by demonstrating the osmosis process by potato osmometer. What is osmolarity?
The Tonicity Riddle: Can You Solve This Solution Mystery?
Osmosis is a passive process and happens without any expenditure of energy. It involves the movement of molecules from a region of higher concentration to...